Types of military rocket fuel
Historical background
Rocket fuel contains fuel and an oxidizing agent and, unlike jet fuel, does not need an external component: air or water. Rocket fuels in their state of aggregation are divided into liquid, solid and hybrid. Liquid fuels are divided into cryogenic (with a boiling point of components below zero degrees Celsius) and high-boiling (the rest). Solid fuels consist of a chemical compound, solid solution or plasticized mixture of components. Hybrid fuels consist of components in a different state of aggregation; at the moment they are at the research stage.

Historically, the first rocket fuel was smoke powder, consisting of a mixture of nitrate (oxidizing agent), charcoal (fuel) and sulfur (binder), which was first used in Chinese rockets in the 2 century A.D. Ammunition with a solid propellant rocket engine (RDTT) was used in the military as an incendiary and signaling device.

After the invention of smokeless gunpowder at the end of the 19th century on its basis, a one-component ballistic fuel was developed, consisting of a solid solution of nitrocellulose (fuel) in nitroglycerin (oxidizing agent). Ballistic fuel has a multiple energy compared to black powder, has high mechanical strength, is well formed, long-term chemical stability during storage, has a low cost. These qualities predetermined the widespread use of ballistic fuel in the most popular munitions equipped with solid propellant rocket propelled rockets - rockets and grenades.
The development of such scientific disciplines as gas dynamics, combustion physics, and the chemistry of high-energy compounds in the first half of the 20th century made it possible to expand the composition of rocket fuels through the use of liquid components. The first Fau-2 liquid-fuel rocket-propelled military rocket engine used a cryogenic oxidizing agent - liquid oxygen and high boiling fuel - ethyl alcohol.
After World War II rocket weapon gained priority in development compared to other types of weapons because of its ability to deliver nuclear charges to the target at any distance - from several kilometers (reactive systems) to intercontinental range (ballistic missiles). In addition, rocket weapons significantly displaced artillery in aviation, Air defense, ground forces and navy due to the lack of recoil when launching ammunition with rocket engines.
At the same time as ballistic and liquid rocket propellants, multicomponent mixed solid fuels developed as the most suitable for military use due to their wide temperature range of operation, elimination of the danger of spillage of components, lower cost of solid propellant rocket engines due to the absence of pipelines, valves and pumps, more traction per unit weight.
Main characteristics of rocket fuels
In addition to the state of aggregation of their components, rocket fuels are characterized by the following indicators:
- specific impulse of thrust;
- thermal stability;
- chemical stability;
- biological toxicity;
- density;
- smokiness.
The specific thrust impulse of rocket fuels depends on the pressure and temperature in the combustion chamber of the engine, as well as the molecular composition of the combustion products. In addition, the specific impulse depends on the degree of expansion of the engine nozzle, but this applies more to the external environment of rocket technology (air atmosphere or outer space).
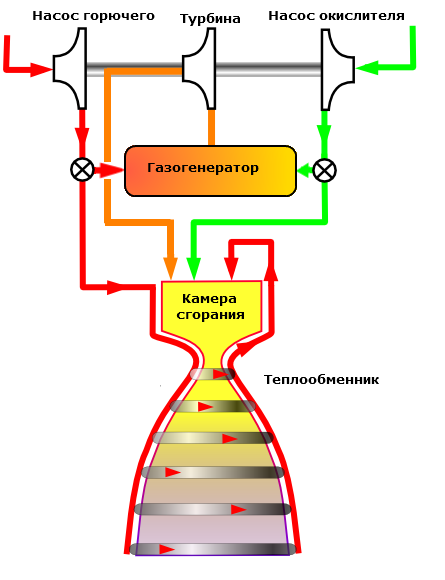
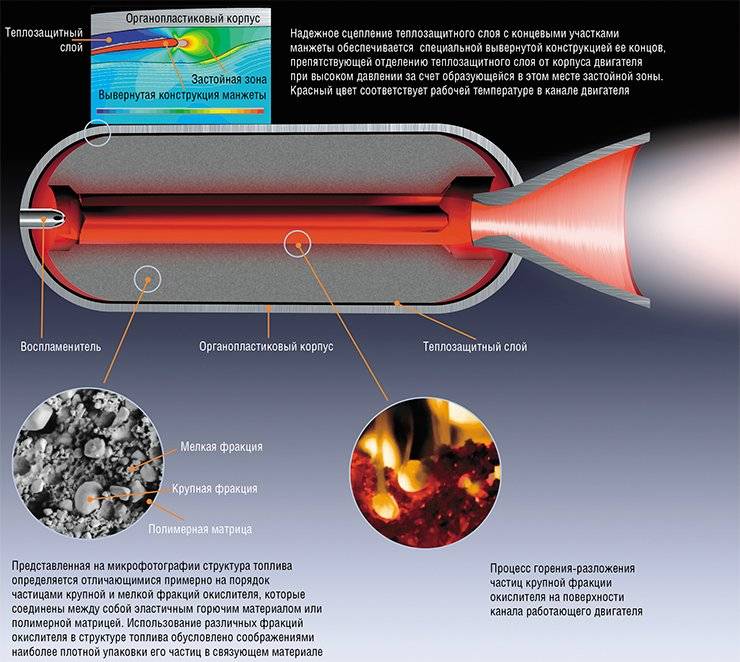
The increased pressure is ensured by the use of structural materials with high strength (steel alloys for rocket engines and organoplastics for solid propellant rocket engines). In this aspect, the liquid propellant rocket engines are ahead of solid propellant rocket engines because of the compactness of their propulsion system compared to the solid-fuel engine housing, which is one large combustion chamber.
The high temperature of the combustion products is achieved by adding aluminum metal to a solid fuel or a chemical compound - aluminum hydride. Liquid fuel can use such additives only if it is thickened with special additives. The thermal protection of the rocket engine is ensured by fuel cooling, the thermal protection of the solid propellant rocket engine is achieved by firmly bonding the fuel block to the walls of the engine and the use of burnable carbon-carbon composite liners in the nozzle critical section.

The molecular composition of the products of combustion / decomposition of fuel affects the flow rate and their state of aggregation at the nozzle exit. The smaller the weight of the molecules, the greater the flow rate: the most preferred combustion products are water molecules, followed by nitrogen, carbon dioxide, chlorine and other halogen oxides; least preferred is aluminum oxide, which condenses in the engine nozzle to a solid state, thereby reducing the volume of expanding gases. In addition, the aluminum oxide fraction forces the use of conical shaped nozzles due to the abrasive wear of the most efficient Laval nozzles with a parabolic surface.
For military rocket fuels, their thermal stability is of particular importance due to the wide temperature range of operation of rocket technology. Therefore, cryogenic liquid fuels (oxygen + kerosene and oxygen + hydrogen) were used only at the initial stage of development of intercontinental ballistic missiles (P-7 and Titan), as well as for space rocket launcher launchers (Space Shuttle and Energy) designed for the launch of satellites and space weapons into near-Earth orbit.
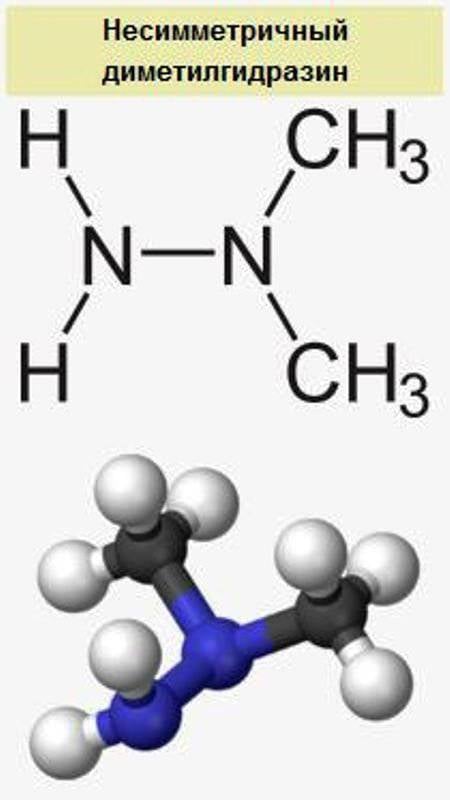
Currently, the military sector uses exclusively high-boiling liquid fuel based on nitrogen tetraoxide (AT, an oxidizing agent) and asymmetric dimethylhydrazine (UDMH, fuel). The thermal stability of this fuel pair is determined by the boiling point of AT (+ 21 ° C), which limits the use of this fuel by missiles located in thermostated conditions of missile silos of ICBMs and SLBMs. Due to component aggressiveness, only one country in the world owned / owns the technology for their production and operation of missile tanks - the USSR / RF (Voevoda and Sarmat ICBMs, Sineva and Liner SLBMs). As an exception, the AT + UDMH is used as the fuel of the X-22 Storm cruise missile, but due to problems with ground operation, the X-22 and their next generation X-32 are planned to be replaced by Zircon cruise missiles using a jet engine using kerosene as a fuel.
The thermal stability of solid fuels is mainly determined by the corresponding property of the solvent and the polymer binder. In the composition of ballistic fuels, the solvent is nitroglycerin, which in the solid solution with nitrocellulose has an operating temperature range from minus to plus 50 ° C. In mixed fuels, various synthetic rubbers with the same operating temperature range are used as a polymer binder. However, the thermal stability of the main components of solid fuels (ammonium dinitramide + 97 ° C, aluminum hydride + 105 ° C, nitrocellulose + 160 ° C, ammonium perchlorate and octogen + 200 ° C) significantly exceeds the similar property of the known binders, and therefore relevant search for their new compounds.
The most chemically stable fuel pair is AT + UDMH, since it developed a unique domestic technology for ampulized storage in aluminum tanks under a slight excess nitrogen pressure for virtually unlimited time. All solid fuels chemically degrade over time due to spontaneous decomposition of polymers and their technological solvents, after which oligomers enter into chemical reactions with other, more stable fuel components. Therefore, solid propellant checkers need regular replacement.
The biologically toxic component of rocket fuels is UDMH, which affects the central nervous system, the mucous membranes of the eyes and digestive tract of a person, and provokes cancer. In this regard, work with UDMH is carried out in insulating suits of chemical protection using self-contained breathing apparatus.
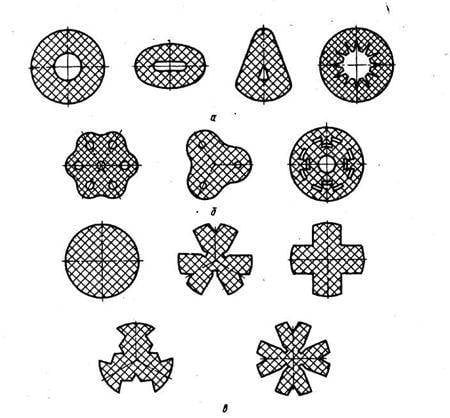
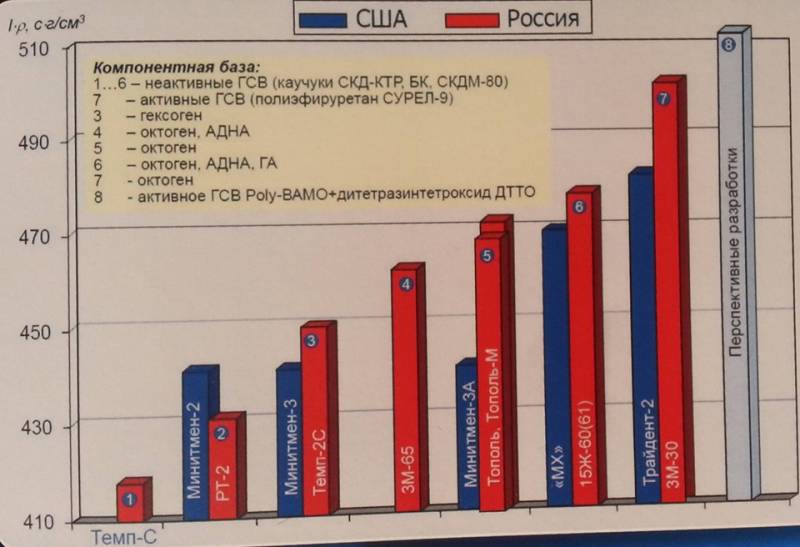
The value of the density of the fuel directly affects the mass of the fuel tanks of the rocket engine and the solid propellant rocket hull: the higher the density, the lower the parasitic mass of the rocket. The lowest density of the hydrogen + oxygen fuel pair is 0,34 g / cu. cm, for a pair of kerosene + oxygen, the density is 1,09 g / cu. cm, AT + UDMH - 1,19 g / cu. cm, nitrocellulose + nitroglycerin - 1,62 g / cu. cm, aluminum / aluminum hydride + ammonium perchlorate / dinitramide - 1,7 g / cc, octogen + ammonium perchlorate - 1,9 g / cc see. It should be borne in mind that axial combustion solid propellant solid propellants have a fuel charge density that is about half the fuel density due to the star-shaped section of the combustion channel used to maintain a constant pressure in the combustion chamber regardless of the degree of fuel burnup. The same applies to ballistic fuels, which are formed in the form of a set of ribbons or checkers to reduce the burning time and acceleration distance of rockets and rockets. In contrast to them, the density of the fuel charge in the solid-propellant solid-propellant solid propellant solid fuel based on HMX coincides with the maximum density indicated for it.
The last of the main characteristics of rocket fuels is the smokiness of the combustion products, visually unmasking the flight of rockets and rockets. This feature is inherent in solid fuels containing aluminum, the oxides of which condense to a solid state during expansion in the nozzle of a rocket engine. Therefore, these fuels are used in solid rocket propulsion ballistic missiles, the active portion of the trajectory of which is outside the direct line of sight of the enemy. Aircraft missiles are equipped with octogen and ammonium perchlorate-based fuels, rockets, grenades and anti-tank missiles with ballistic fuels.
Rocket Fuel Energy
To compare the energy capabilities of different types of rocket fuel, it is necessary to set for them comparable combustion conditions in the form of pressure in the combustion chamber and the degree of expansion of the rocket engine nozzle - for example, 150 atmospheres and 300-fold expansion. Then, for fuel pairs / triples, the specific impulse will be:
oxygen + hydrogen - 4,4 km / s;
oxygen + kerosene - 3,4 km / s;
AT + UDMH - 3,3 km / s;
ammonium dinitramide + hydrogen hydride + octogen - 3,2 km / s;
ammonium perchlorate + aluminum + octogen - 3,1 km / s;
ammonium perchlorate + octogen - 2,9 km / s;
nitrocellulose + nitroglycerin - 2,5 km / s.
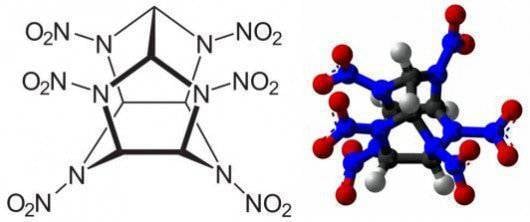
Solid fuel based on ammonium dinitramide was a domestic development of the late 1980-ies, was used as fuel of the second and third stages of the RT-23 UTTX and P-39 rockets and has still not been surpassed in energy performance by the best samples of foreign fuel based on ammonium perchlorate, used in Minuteman-3 and Trident-2 missiles. Ammonium dinitramide is an explosive that detonates even from light radiation; therefore, it is produced in rooms lit by low-power red light lamps. Technological difficulties did not allow to master the process of manufacturing rocket fuel based on it anywhere in the world, except in the USSR. It’s another matter that Soviet technology was routinely implemented only at the Pavlograd Chemical Plant, located in the Dnepropetrovsk Region of the Ukrainian SSR, and was lost in the 1990 years after the plant was reprofiled to produce household chemicals. However, judging by the tactical and technical characteristics of promising models of weapons of the RS-26 "Frontier" type, the technology was restored in Russia in the 2010-s.
An example of a highly effective composition is the composition of solid rocket fuel from Russian patent No. 2241693 owned by the Federal State Unitary Enterprise Perm Plant named after CM. Kirov ":
the oxidizing agent is ammonium dinitramide, 58%;
fuel - aluminum hydride, 27%;
plasticizer - nitroisobutyl trinitrate glycerol, 11,25%;
binder - polybutadiene nitrile rubber, 2,25%;
hardener - sulfur, 1,49%;
combustion stabilizer - ultrafine aluminum, 0,01%;
additives - soot, lecithin, etc.
Prospects for the development of rocket fuels
The main areas of development of liquid rocket fuels are (in order of priority):
- the use of supercooled oxygen in order to increase the density of the oxidizing agent;
- the transition to a fuel pair of oxygen + methane, the combustible component of which has 15% more energy and 6 times better heat capacity than kerosene, given that aluminum tanks are hardened at the temperature of liquid methane;
- the addition of ozone to the composition of oxygen at the level of 24% in order to increase the boiling point and energy of the oxidizing agent (a large proportion of ozone is explosive);
- the use of thixotropic (thickened) fuel, the components of which contain suspensions of pentaborane, pentafluoride, metals or their hydrides.
Supercooled oxygen is already used in the Falcon 9 launch vehicle; oxygen + methane fuel-oil rocket engines are being developed in Russia and the USA.
The main direction of development of solid rocket fuels is the transition to active binders containing oxygen in their molecules, which improves the oxidative balance of solid fuel as a whole. The modern domestic example of such a binder is the Nika-M polymer composition, which includes cyclic groups of dinitrile dioxide and butylenediol polyether urethane, developed by the State Research Institute of Crystal (Dzerzhinsk).
Another promising area is the expansion of the range of nitramine explosives used, which have a greater oxygen balance compared to octogen (minus 22%). First of all, it is hexanitrohexaazaisowurtzitane (Cl-20, oxygen balance minus 10%) and octanitrocubane (zero oxygen balance), the prospects of their use depend on lowering the cost of their production - currently Cl-20 is much more expensive than octogen, octonitrocuban is much more expensive than Cl -20.
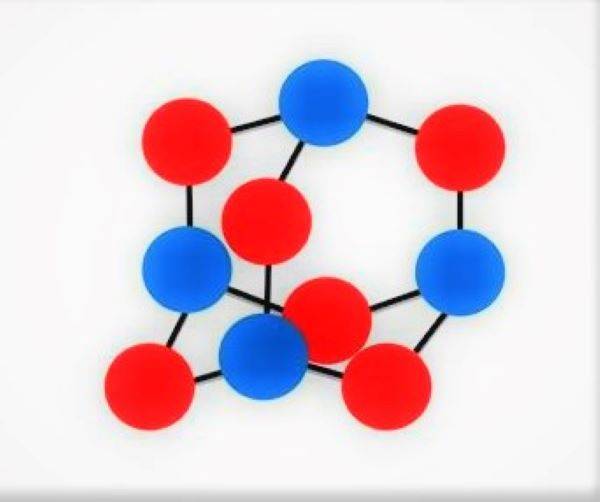
In addition to improving the known types of components, research is also being carried out in the direction of creating polymer compounds whose molecules consist exclusively of nitrogen atoms interconnected by single bonds. As a result of decomposition of the polymer compound under the influence of heating, nitrogen forms simple molecules of two atoms connected by a triple bond. The energy released in this case is twice the energy of nitramine explosives. For the first time, nitrogen compounds with a diamond-like crystal lattice were obtained by Russian and German scientists in 2009 during experiments on a joint experimental setup under the influence of pressure in 1 million atmospheres and temperature in 1725 ° C. Currently, work is underway to achieve a metastable state of nitrogen polymers at ordinary pressures and temperatures.
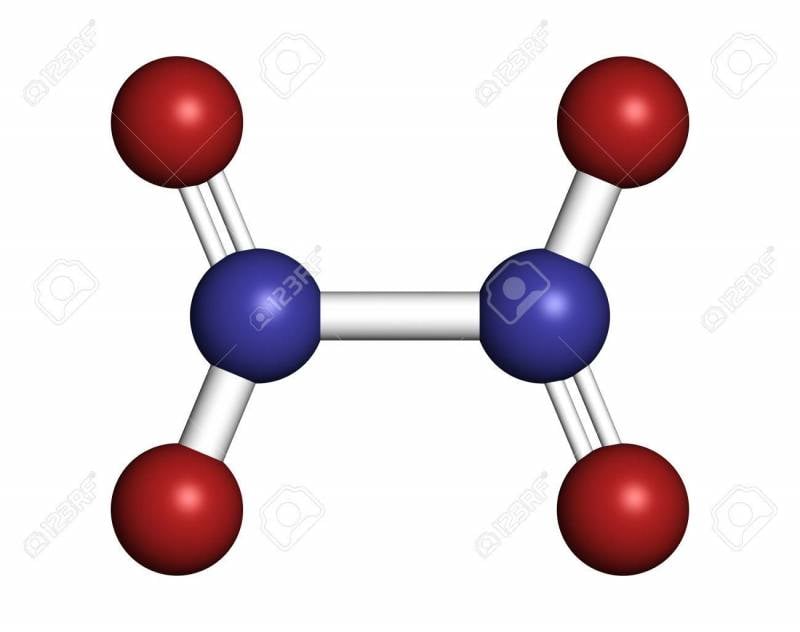
Promising oxygen-containing chemical compounds are higher nitrogen oxides. Known nitric oxide V (a planar molecule of which consists of two nitrogen atoms and five oxygen atoms) does not represent practical value as a component of solid fuel due to its low melting point (32 ° C). Research in this direction is being carried out by searching for a method for the synthesis of nitric oxide VI (tetraazotone hexoxide), the skeleton molecule of which is in the form of a tetrahedron, at the tops of which there are four nitrogen atoms linked to six oxygen atoms located on the edges of the tetrahedron. The complete closure of the interatomic bonds in the molecule of nitric oxide VI makes it possible to predict for it increased thermal stability similar to urotropine. The oxygen balance of nitric oxide VI (plus 63%) allows you to significantly increase the specific gravity of solid rocket fuels of such high-energy components as metals, metal hydrides, nitramines and hydrocarbon polymers.


Information