Nitrates in the war. Part I. From Sun-Symyao and Bertold Schwartz to D.I. Mendeleev
In preparing the outline of the lessons, the author repeatedly noted that the countries whose rulers gave vigilant attention to the development of sciences, and above all - the natural trinity of mathematics - physics - chemistry - reached heights in their development. A vivid example is the rapid ascent on the world stage of Germany, which in half a century made a leap from the union of disparate states, some of which even on a detailed map of Europe could not be seen without a small-scale scope, to an empire that had to be reckoned for over a century and a half. Without diminishing the merit of the great Bismarck in this process, I’ll quote his phrase he said after the victorious conclusion of the Franco-Prussian war: "A simple German teacher won this war." The author would like to devote his review to the chemical aspect of enhancing the combat effectiveness of the army and the state, as always, without pretending to the exclusivity of his opinion.
By publishing the article, the author deliberately, like Jules Verne, avoids specifying specific technological details and focuses on purely industrial methods for obtaining explosives. This is connected not only with the scientist’s understandable sense of responsibility for the results of his works (whether practical or journalistic), but also because the subject of the research is the question “Why it was so and not otherwise” and not “Who first got it substance".
In addition, the author apologizes to readers for the forced use of chemical terms - attributes of science (as shown by their own teaching experience, not the most beloved by schoolchildren). Realizing that it is impossible to write about chemicals, without mentioning chemical terms, the author will try to minimize specific vocabulary.
And the last. The figures cited by the author should not be considered the ultimate truth. Data on the characteristics of explosives in different sources are different and sometimes quite strong. This is understandable: the characteristics of ammunition greatly depend on their “commercial” type, the presence / absence of foreign substances, the introduction of stabilizers, the modes of synthesis, and many other factors. Methods for determining the characteristics of explosives also do not differ in uniformity (although there will be more standardization here) and they do not suffer from special reproducibility either.
BB classification
Depending on the type of explosion and sensitivity to external influences, all explosives are divided into three main groups:
1. Initiating BB.
2. Blasting explosives.
3. Throwing explosives.
Initiating BB. They are highly sensitive to external influences. The remaining characteristics of them are usually low. But they have a valuable property - their explosion (detonation) has a detonation effect on blasting and propelling explosives, which are usually not sensitive at all to other types of external influences or have very low sensitivity. Therefore, initiating substances are used only for initiating an explosion of blasting or propelling explosives. To ensure the safety of initiating explosives, they are packed in protective devices (capsule, capsule sleeve, detonator capsule, electric detonator, fuse). Typical representatives of initiating explosives are: explosive mercury, lead azide, teneres (TNRS).
Blasting explosives. This, in fact, is what they say and write about. They equip shells, mines, bombs, rockets, land mines; they blow up bridges, cars, businessmen ...
Blasting explosives according to their explosive characteristics are divided into three groups:
- increased power (representatives: RDX, HKT, ten, tetryl);
- normal power (representatives: TNT, Melinit, plasticite);
- reduced power (representatives: ammonium nitrate and its mixtures).
Explosives of increased power are somewhat more sensitive to external influences and therefore they are more often used in a mixture with phlegmatizers (substances that lower the sensitivity of explosives) or in a mixture with explosives of normal power to increase the power of the latter. Sometimes high power explosives are used as intermediate detonators.
Throwing explosives. These are various gunpowder - black smoky, smokeless pyroxylin and nitroglycerin. They also include various pyrotechnic mixtures for fireworks, signal and lighting rockets, lighting projectiles, mines, and aerial bombs.
About black powder and black berthold
For several centuries, the only kind of explosive that was used by man was black powder. With it, they threw nuclei at the enemy from cannons, filling explosive shells with them. Gunpowder was used in underground mines for the destruction of the walls of fortresses, for crushing rocks.
In Europe, he became known from the XIII century, and in China, India and Byzantium even earlier. The first recorded description of gunpowder for fireworks was described by the Chinese scientist Sun-Shymyao in 682, Maximilian Grek (XIII-XIV centuries) in his treatise "Book of lights" described a mixture based on potassium nitrate used in Byzantium as the famous "Greek fire" and consisting from 60% saltpeter, 20% sulfur and 20% coal.
 The European history of gunpowder discovery begins with an Englishman, a Franciscan monk Roger Bacon, who in 1242 year in his book Liber de Nullitate Magiae gives a recipe for black powder for rockets and fireworks (40% saltpeter, 30% coal and 30% sulfur) and the semi-mythical monk Burt. Schwarz (1351 year). However, it is possible that this was one person: the use of pseudonyms in the Middle Ages was quite common, as was the subsequent confusion with the dating of sources.
The European history of gunpowder discovery begins with an Englishman, a Franciscan monk Roger Bacon, who in 1242 year in his book Liber de Nullitate Magiae gives a recipe for black powder for rockets and fireworks (40% saltpeter, 30% coal and 30% sulfur) and the semi-mythical monk Burt. Schwarz (1351 year). However, it is possible that this was one person: the use of pseudonyms in the Middle Ages was quite common, as was the subsequent confusion with the dating of sources.The simplicity of the composition, the availability of two of the three components (native sulfur and now not uncommon in southern Italy and Sicily), ease of preparation - all this guaranteed a powder triumphal procession in Europe and Asia. The only problem was obtaining large quantities of potassium nitrate, but they successfully coped with this task. Since the only known potassium nitrate deposit at that time was in India (hence its second name is Indian), local production was established in almost all countries. It was impossible to call it pleasant, even with a solid reserve of optimism: raw materials for it were manure, animal insides, urine and animal hair. The least unpleasant components of this foul-smelling and highly soiled mixture were lime and potash. All this wealth for several months fell into the pits, where it wandered under the action of azotobacteria. The ammonia released was oxidized to nitrates, which ultimately yielded coveted nitrate, which was isolated and purified by recrystallization — the job, I’m also saying, is not the most pleasant. As you can see, there is nothing particularly difficult in the process, the raw material is quite affordable and the availability of gunpowder also soon became universal.
Black (or smoky) powder at that time was a universal explosive. Neither shaky, nor roll, for many years it was used both as a throwing tool and as a filling for the first bombs - types of modern ammunition. Until the end of the first third of the XIX century, the powder fully met the needs of progress. But science and industry did not stand still, and soon it ceased to meet the requirements of the time because of its small capacity. The end of the monopoly of gunpowder can be attributed to the 70 years of the 17th century, when A. Lavoisier and C. Berthollet organized the production of bertolet salt based on the open potassium chlorate (bertolet salt) discovered by Bertholl.
The history of bertolet salt can be started from the moment when Claude Berthollet studied the properties of chlorine recently discovered by Karl Scheele. By passing chlorine through a hot concentrated solution of potassium hydroxide, Berthollet obtained a new substance, later called chemists by potassium chlorate, and not by chemists, by the berthollet salt. It happened in 1786 year. And although the devil's salt did not become a new explosive, it fulfilled its role: first, it served as an incentive to search for new substitutes by the order of the decrepit "god of war", and second, it became the ancestor of new types of explosives - initiators.
Explosive oil

And in 1846, the chemists proposed two new explosives - pyroxylin and nitroglycerin. In Turin, the Italian chemist Askanio Sobrero discovered that it was enough to process glycerin with nitric acid (perform nitration) to form an oily transparent liquid — nitroglycerin. The first printed message about him was published in the journal L'Institut (XV, 53) from 15 February 1847, and it deserves some citation. The first part says:
Next comes a description of the experience of nitration, which is interesting only to organic chemists (and only from a historical point of view), we will only note one feature: the nitro derivatives of cellulose, like their ability to explode, were also quite well known [11].
Nitroglycerin is one of the most powerful and sensitive blasting explosives, the treatment of which requires special care and caution.
1. Sensitivity: from lumbago bullet may explode. Sensitivity to impact 10 kg weight, dropped from a height 25 cm - 100%. Burning goes into detonation.
2. The energy of the explosive transformation - 5300 J / kg.
3. Knock speed: 6500 m / s.
4. Brizantnost: 15-18 mm.
5. Explosiveness: 360-400 cube. see [6].
The use of nitroglycerin was demonstrated by the famous Russian chemist NN Zinin, who in 1853 — 1855 during the Crimean War, together with military engineer VF Petrushevsky, produced a large amount of nitroglycerin.

Professor of Kazan University N.N. Zinin

Military engineer V.F. Petrushevsky
But the devil, who lives in nitroglycerin, turned out to be malicious and rebellious. It turned out that the sensitivity of this substance to external influences is only slightly inferior to explosive mercury. It can explode at the time of nitration, it can not be shaken, heated and cooled, put up in the sun. It may explode during storage. And if you set it on fire with a match, it can burn completely calmly ...
 Yet the need for powerful explosives by the middle of the XIX century was already so great that, despite numerous accidents, nitroglycerin was widely used in blasting operations.
Yet the need for powerful explosives by the middle of the XIX century was already so great that, despite numerous accidents, nitroglycerin was widely used in blasting operations.Attempts to curb the evil devil made many, but the glory tamer got Alfred Nobel. The ups and downs of this journey, as well as the fate of the proceeds from the sale of this substance, are quite widely known, and the author considers it unnecessary to go into their details.
Being “squeezed” into the pores of an inert filler (several dozens of substances were tried, the best of which was infusor earth - porous silicate, 90% of which falls into pores capable of absorbing nitroglycerin eagerly), retaining much more “compliant” carrying almost all of its destructive power. As is known, Nobel gave this mixture, externally similar to peat, the name "dynamite" (from the Greek word "dinos" - force). The irony of fate: a year after Nobel received the patent for the production of dynamite, Petrushevsky completely independently mixed nitroglycerin with magnesia and received explosives, later called “Russian dynamite”.
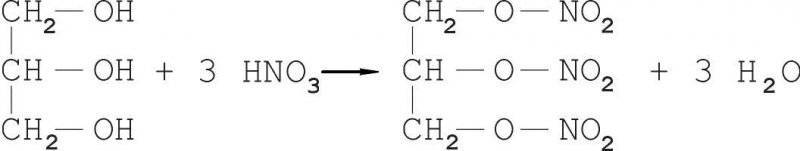
Nitroglycerin (more precisely, glycerol nitrile) is a complete ester of glycerol and nitric acid. It is usually obtained by treating glycerol with a sulfur-nitric acid mixture (in a chemical language, the esterification reaction):
The explosion of nitroglycerin is accompanied by the release of a large amount of gaseous products:
The esterification proceeds sequentially in three steps: in the first one glycerol mononitrate is obtained, in the second one - glycerol dinitrate and in the third one - glycerol nitrite. For a more complete yield of nitroglycerin, take 20% excess of nitric acid in excess of the theoretically required amount.
Nitration was carried out in porcelain pots or soldered lead vessels standing in an ice-water bath. In one run, about 700 g of nitroglycerin was obtained, and within an hour of such operations it was performed on 3 - 4.
But the growing needs have made their adjustments to the technology of nitroglycerin. Over time (in 1882), the technology for producing explosives in nitrators was developed. The process was divided into two stages: at the first stage, glycerin was mixed with half the amount of sulfuric acid, and thus most of the generated heat was utilized, after which a ready mixture of nitric and sulfuric acids was injected into the same vessel. Thus, the main difficulty was avoided: excessive overheating of the reaction mixture. Mixing is performed with compressed air under pressure 4 atm. Process performance - 100 kg of glycerin per 20 min at 10 - 12 degrees.
Due to the different specific weight of nitroglycerin (1,6) and spent acid (1,7), it is collected from above with a sharp interface. After nitration, nitroglycerin is washed with water, then washed with acid from the residue with soda and washed again with water. Mixing at all stages of the process is performed with compressed air. Drying is performed by filtration through a layer of calcined table salt [9].
As you can see, the reaction is quite simple (remember the wave of terrorism at the end of the 19th century, raised by “bombers” who mastered the simple science of applied chemistry) and is one of the “uncomplicated chemical processes” (A. Stetbacher). It is possible to make almost any amount of nitroglycerin in the simplest conditions (it is not much easier to make black powder).
The consumption of reagents is as follows: to obtain 150 ml of nitroglycerin, you need to take: 116 ml of glycerin; 1126 ml of concentrated sulfuric acid;
649 ml of nitric acid (at least 62% concentration).
Dynamite in war
 Dynamite was first used in the Franco-Prussian War of 1870 — 1871: Prussian sappers blew dynamite with French fortifications. But the safety of dynamite turned out to be relative. The military immediately found out that when a bullet is fired by a bullet, it explodes no worse than its progenitor, and burning in certain cases turns into an explosion.
Dynamite was first used in the Franco-Prussian War of 1870 — 1871: Prussian sappers blew dynamite with French fortifications. But the safety of dynamite turned out to be relative. The military immediately found out that when a bullet is fired by a bullet, it explodes no worse than its progenitor, and burning in certain cases turns into an explosion.But the temptation to get powerful ammunition was irresistible. Through quite dangerous and complex experiments, we managed to find out that dynamite would not detonate if the loads would increase not instantaneously, but gradually, keeping the acceleration of the projectile in a safe framework.
The solution to the problem at the technical level was seen in the use of compressed air. In June, Lt. X. NUMX, Lieutenant Edmund Ludwig G. Zelinsky of the United States Army’s 1886 Artillery Regiment, conducted tests and refined the original design of American Engineering. A pneumatic gun with a caliber of 5 mm and a length of 380 m using air compressed to 15 atm could throw projectiles of length 140 m with 3,35 kg of dynamite on 227 m. And a projectile of length 1800З m with 1,8 kg of dynamite and all 51 kms.
The driving force was provided by two compressed air cylinders, the upper one of which was connected to the implement with a flexible hose. The second cylinder was a reserve for the power supply of the upper one, and the pressure in it was maintained by means of a steam pump buried in the ground. Launched with dynamite, the projectile had the form of a darth — an artillery boom — and had an 50-pound warhead.
The Duke of Cambridge ordered the army to test one such system at Milford Haven, but the cannon spent almost all the ammunition before it finally hit the target, which, however, was destroyed very effectively. The American admirals were delighted with the new cannon: in 1888, money was released to make 250 dynamite guns for coastal artillery.

In 1885, Zelinsky established the Pneumatic Gun Company for deployment in the army and navy pneumatic guns with dynamite shells. His experiments made us talk about air guns as a new promising weapons. The US Navy even built a Vesuvius dynamite cruiser with a displacement of 1888 tons in 944, armed with three such 381 mm caliber guns.
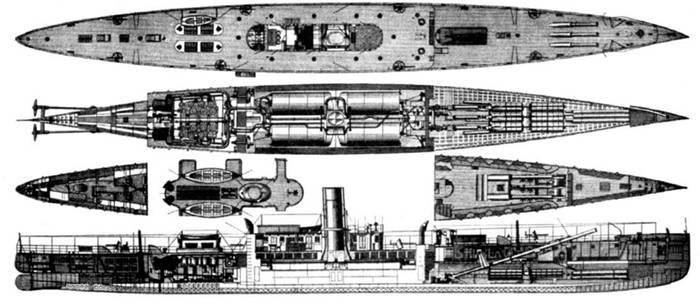
The scheme of the "dynamite" cruiser "Vesuvius"
[Center]
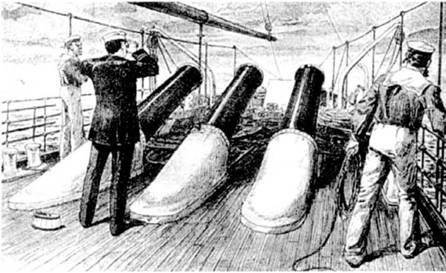
And so it looked outward motionless guns[/ Center]
But a strange thing: a few years later, enthusiasm was replaced by disappointment. “During the Spanish-American war,” the American artillerymen spoke about this, “these guns never hit the right place.” And although the point here was not so much the guns, but the ability of the artillerymen to shoot straight and the rigid attachment of guns, this system did not receive further development.
In 1885, Holland installed the Zelinsky airgun on his submarine number 4. However, it did not come to its practical tests, since the boat suffered a serious accident during the launch.
In 1897, Holland re-armed his submarine No. 8 with the new Zelinsky gun. The armament was presented with a nose torpedo tube caliber 18 inches (457 mm) with three Whitehead torpedoes, as well as Zelinsky stern airgun for dynamite shells (7 ammunition load of 222 kg) each. However, due to the barrel being too short, limited by the size of the boat, this gun had a small firing range. After practical firing, the inventor dismantled it in 100,7.
In the future, neither Holland nor other designers installed guns (apparatus) for firing throwing mines and dynamite shells on their submarines. So Zelinsky's guns imperceptibly, but quickly disappeared from the scene [12].
Nitroglycerin sibling
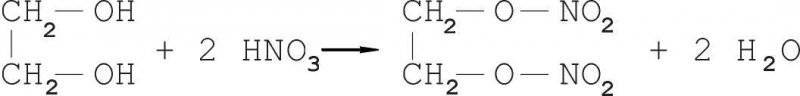
From a chemical point of view, glycerin is the simplest representative of the class of triatomic alcohols. There is its diatomic counterpart - ethylene glycol. Is it any wonder that after they got acquainted with nitroglycerin, chemists also paid attention to ethylene glycol, hoping that it would be more convenient to use.
But here again the devil of explosives showed his capricious character. The characteristics of dinitroethylene glycol (it never received its own name) were not much different from nitroglycerin:
1. Sensitivity: detonation when 2 is dropped kg load from height 20 cm; sensitive to friction, fire.
2. The energy of the explosive transformation - 6900 J / kg.
3. Knock speed: 7200 m / s.
4. Brizantnost: 16,8 mm.
5. Explosiveness: 620 — 650 cube. cm.
For the first time, Henry was obtained in 1870 g. It is obtained by careful nitration of ethylene glycol in a manner similar to the production of nitroglycerin (a nitrating mixture: H2SO4 - 50% HNO3 - 50%; ratio - 1 to 5 with respect to ethylene glycol).
The nitration process can be conducted at a lower temperature, which is a predisposition to a higher yield [7, 8].
Despite the fact that, in general, the sensitivity of DNEG was slightly lower than that of NG, its application did not promise significant benefits. If we add to this even higher than that of NG, volatility and lower availability of raw materials, then it becomes clear that this path also led nowhere.
However, he didn’t turn out to be completely useless either. Initially, it was used as an additive to dynamite, during the Second World War, due to a lack of glycerol, was used as a substitute for nitroglycerin in smokeless powders. Such gunpowder had a short shelf life due to the volatility of the DNEG, but in wartime conditions it did not matter: for a long time no one was going to store them.
Christian Schönbein Apron
It is not known how long the military would have spent searching for ways to calm nitroglycerin, if by the end of the 19th century the industrial technology of obtaining another nitroether had not arrived. In short, the story of his appearance is as follows [16].
In 1832, the French chemist Henri Brakonne discovered that the processing of starch and wood fibers with nitric acid produced an unstable combustible and explosive material, which he called xyloidin. However, the case of this discovery and limited to a message. Six years later, in 1838, another French chemist, Theophile-Jules Pelouse, processed paper and paperboard in a similar way and obtained similar material, which he called nitramidine. Who would have thought then, but the reason for the impossibility of using nitramidine for technical purposes was precisely its low stability.
In the 1845 year, the Swiss chemist Christian Friedrich Schönbein (who was famous for his discovery of ozone by that time) was conducting experiments in his laboratory. His wife strictly forbade him to bring his flasks to the kitchen, so he was in a hurry to finish the experiment in her absence - and he spilled some tart mixture on the table. In an effort to avoid scandal, he, in the best tradition of Swiss accuracy, wiped it with his working apron, the benefit of the mixture was not too much. Then, also in the tradition of Swiss frugality, he washed the apron with water and hung it up to dry over the stove. Whether he was hanging there for a short time, history is silent, but the fact that the apron suddenly disappeared after drying has been known for certain. Moreover, it did not disappear quietly, in English, but loudly, you can even say enchantingly: in a flash and a loud bang of an explosion. But what attracted Schönbein's attention: the explosion occurred without the slightest trickle of smoke!
And although Schönbein was not the first to discover nitrocellulose, it was for him to conclude that it was important to discover. At that time, black powder was used in artillery, the soot from which soiled the guns that in the intervals between shots they had to be cleaned, and after the first volleys such a curtain of smoke was raised that it was necessary to fight almost blindly. What is already to say that the clouds of black smoke perfectly marked the location of the batteries. The only thing that brightened life - is the realization that the enemy was in the same position. Therefore, to the explosive substance, which gives much less smoke, and besides also more powerful than black powder, the military reacted with enthusiasm.
Nitrocellulose, devoid of the disadvantages of black powder, allowed to start production of smokeless powder. And, in the traditions of that time, it was decided to use it both as a propellant and as an explosive. In the 1885 year, after numerous experimental work, the French engineer Paul Viel received and tested several kilograms of pyroxylin plate powder, called the powder "B" - the first smokeless powder. Tests have proven the benefits of a new powder.
However, it was not easy to manufacture large quantities of nitrocellulose for military needs. Nitrocellulose was too impatient to wait for battles and factories, as a rule, with enviable regularity took off into the air, as if competing in this with nitroglycerin production. When creating the industrial production technology of pyroxylin, such obstacles had to be overcome as for no other explosive. It took an entire quarter of a century to carry out a number of works by researchers from different countries, until this original fiber explosive became suitable for use and until numerous means and methods were found that were somehow safe from an explosion during prolonged storage of the product. The expression "any" is not a literary device, but a reflection of the difficulty that chemists and technologists have encountered in defining sustainability criteria. Hard judgments about the approaches to determining the criteria of stability were not, and with further expansion of the use of this explosive constant explosions revealed more and more mysterious features in the behavior of this peculiar ester. Only in 1891, James Dewar and Frederick Abel managed to find a safe technology.
Production of pyroxylin requires a large number of auxiliary devices and a long process in which all operations must be carried out equally thoroughly and thoroughly.
The starting material for the production of pyroxylin is cellulose, the best representative of which is cotton. Natural pure cellulose is a polymer consisting of glucose residues, being a close relative of starch:(C6H10O5)n. In addition, waste from a cotton mill can be a source of excellent raw materials.
Fiber nitration was mastered on an industrial scale as far back as the 60 of the 19th century and was carried out in ceramic pots with further spinning in centrifuges. However, by the end of the century, this primitive method was supplanted by American technology, although it was revived during the years of the WWI due to its low cost and simplicity (more precisely, primitivism).
The purified cotton is loaded into the nitrator, a nitrating mixture is added (HNO3 - 24%, H2SO4 - 69%, water - 7%) based on 15 kg of fiber 900 kg of the mixture, which gives the output 25 kg of pyroxylin.
Nitrators are connected to batteries consisting of four reactors and one centrifuge. Nitrator loading is performed at a time interval (approximately 40 min), equal to the spin time, which ensures the continuity of the process.
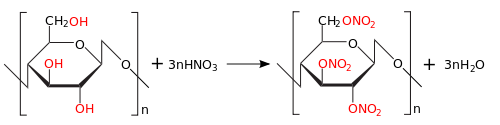
Pyroxylin is a mixture of products of varying degrees of nitration of cellulose. Pyroxylin, obtained when using phosphoric acid instead of sulfuric acid, is highly stable, but this technology has not taken root due to its higher cost and lower productivity.
Pressed pyroxylin tends to spontaneously ignite and needs moisture. The water used for washing and stabilization of pyroxylin should not contain alkaline agents, since alkaline degradation products are catalysts of self-ignition. Final drying to the required moisture is achieved by flushing with absolute alcohol.
But moisturized nitrocellulose is not free from trouble: it is susceptible to infection by microorganisms that cause the appearance of mold. Protect it by waxing the surface. The finished product had the following characteristics:
1. The sensitivity of pyroxylin is highly dependent on moisture. Dry (3 - 5% moisture) easily ignites from an open flame or a touch of hot metal, drilling, friction. It explodes from falling loads 2 kg from a height 10 cm. With increasing humidity, the sensitivity decreases and with 50% water the detonation ability disappears.
2. The energy of explosive transformation - 4200 MJ / kg.
3. Knock speed: 6300 m / s.
4. Brizantnost: 18 mm.
5. Explosiveness: 240 cube cm.
And yet, despite the shortcomings, the chemically more stable pyroxylin suited the military more than nitroglycerin and dynamite, its sensitivity could be adjusted by changing its humidity. Therefore, pressed pyroxylin began to find widespread use for equipping warheads of mines and shells, but over time, this unparalleled product gave way to a nitrated derivative of aromatic hydrocarbons. Nitrocellulose remained as a throwing explosive, but as a blasting explosive it was forever a thing of the past [9].
Snake and nitroglycerin powder
The reader, at least a little familiar with the history of chemistry, has probably already guessed whose words are the genius Russian chemist D.I. Mendeleev.

As a field of chemical knowledge, Mendeleev devoted a lot of his strength and attention to the last years of his life - in 1890 — 1897. But, as always, the active phase of development was preceded by a period of reflection, accumulation and systematization of knowledge.
It all started with the fact that in 1875, the tireless Alfred Nobel made another discovery: a plastic and elastic solid solution of nitrocellulose in nitroglycerin. It quite successfully combined solid form, high density, ease of molding, concentrated energy and insensitivity to the high humidity of the atmosphere. The jelly, completely burned into carbon dioxide, nitrogen and water, consisted of 8% of dinitrocellulose and 92% of nitroglycerin.
Unlike Nobel Tech, D.I. Mendeleev proceeded from a purely scientific approach. The basis of his research, he put a well-defined and strictly chemically sound idea: the desired substance during combustion should produce a maximum of gaseous products per unit of weight. From a chemical point of view, this means that oxygen in this compound should be sufficient to completely convert carbon to gaseous oxide, hydrogen to water, and oxidizing ability to provide energy for the whole process. A detailed calculation led to the formula of the following composition: C30Н38(I have not2)12O25. When burning should get the following:
To carry out a purposeful reaction of synthesizing a substance of such a composition even at the present time is not an easy task, therefore, a mixture of 7 - 10% nitrocellulose and 90 - 93% nitroglycerin was used in practice. The percentage of nitrogen is about 13,7%, which is slightly higher than pyrocollodion (12,4%). The operation is not particularly difficult, does not require the use of complex equipment (carried out in the liquid phase) and proceeds under normal conditions.
In 1888, Nobel received a patent for gunpowder from nitroglycerin and colloxylin (low-fiber), called smokeless like pyroxylin powder. This composition is almost unchanged to date under various technical names, the most famous of which are cordite and ballistitis. The main difference is in the ratio between nitroglycerin and pyroxylin (it has more in cordite) [13].
How do these explosives relate to each other? Refer to the table:
---------------------------------------------------------------------------------
BB ...... Sensitivity .... Energy ... Speed ...... Brisance ... High Duty
......... (kg / cm /% of explosions) .... explosion .... detonation
--------------------------------------------------------------------------------------
ГН..........2/4/100............5300........6500...........15 - 18...........360 - 400
ДНЭГ......2/10/100...........6900.........7200..........16,8...............620 - 650
НК.........2/25/10............4200.........6300...........18.................240
--------------------------------------------------------------------------------------
The characteristics of all explosives are quite close, but the differences in physical properties dictated different niches of their use.
As we have already seen, neither nitroglycerin nor pyroxylin pleased the military with its character. The reason for the low stability of these substances, it seems to me, lies on the surface. Both compounds (or three - counting and dinitroethylene glycol) are representatives of the class of esters. And the ester grouping is not among the leaders of chemical resistance. Rather, it can be found among outsiders. A nitro group containing nitrogen in a rather strange oxidation state for it + 5 is also not a stability sample. The symbiosis of this strong oxidizing agent with such a good reducing agent as the hydroxyl group of alcohols inevitably leads to a number of negative consequences, the most unpleasant of which is capriciousness in application.
Why did chemists and military spend so much time experimenting with them? As it seems, many things bribed many. Military - high power and availability of raw materials, which increased the combat capability of the army and made it insensitive to military units in wartime. Technologists - mild conditions of synthesis (no need to use high temperatures and high pressure) and technological convenience (despite the multistage processes, all reactions take place in the same reaction volume and without the need to isolate intermediate products).
Practical yields of products were also quite high (Table 2), which did not cause an urgent need to search for sources of large amounts of cheap nitric acid (with sulfuric acid the issue was resolved much earlier).
-----------------------------------------------------------------------------------
BB ...... Consumption of reagents on 1 kg ..... Number of stages .... Number of allocated products
......... Nitrogen to-ta..Serna to-that
-----------------------------------------------------------------------------------
GN ....... 10 ................. 23 ................. 3 ..... ................... 1
DNEG .... 16,5 .............. 16,5 ............... 2 ............. ........... 1
NC ........ 8,5 ............... 25 ................. 3 ...... .................. 1
-----------------------------------------------------------------------------------
The situation has changed a lot when new incarnations of the devil of explosives appeared on the scene: trinitrophenol and trinitrotoluene.
Information